To become a licensed pharmacist in California, candidates must pass the North American Pharmacist Licensure Examination® (NAPLEX®) and the California Practice Standards and Jurisprudence Examination for Pharmacists (CPJE). The California State Board of Pharmacy developed the CPJE to assess a candidate's ability to practice pharmacy safely and effectively while adhering to California-specific laws. This article acts as a broad introduction to what candidates should expect as they continue their journey to becoming licensed pharmacists in California.
Why Take the CPJE & Who Can Take It?
To practice as a licensed pharmacist in the state of California, individuals must pass the CPJE. Even if you have taken the Multistate Pharmacy Jurisprudence Examination (MPJE) in another state, you are not eligible for pharmacist licensure in California without first passing the CPJE.
To register and sit for the CPJE, candidates must be a graduate of a pharmacy school accredited by the Accreditation Council for Pharmacy Education® (ACPE®) or have received certification from the Foreign Pharmacy Graduate Examination Committee® (FPGEC®). Applicants who have graduated on or after January 1, 2016 from an ACPE-accredited school of pharmacy are deemed to have satisfied the pharmacy practice experience requirements and are not required to submit pharmacy intern hours. Graduates of a foreign pharmacy school or those that graduated prior to January 1, 2016 must provide documentation of 1,500 hours of pharmacy practice experience as an intern pharmacist, unless licensed as a pharmacist for at least one year in another state.
CPJE Format and Structure

Examinees are provided 15 minutes to complete an introductory tutorial before their exam begins, which does not subtract from the total exam time. Questions test a candidate's knowledge and ability to make educated pharmaceutical decisions while adhering to California Law. After a question is answered, examinees must proceed to the next question and are unable to return to previously answered questions. There is no penalty for guessing, so it is recommended to attempt every question.
Arrive 30 minutes before the scheduled exam to allow time for identification verification. Once the registration process begins, examinees cannot leave until they have completed their exam. Restroom breaks are permitted, but a regulatory entity will be notified if the examinee takes longer than 5 minutes. Note that the exam clock will not pause during breaks and will continue to count down.
CPJE test dates
Below are the CPJE exam dates for 2025. If a candidate fails the CPJE and needs to retake it, they must wait 45 days.
| 2025 CPJE Exam Dates |
|---|
|
What Is On The CPJE Exam?
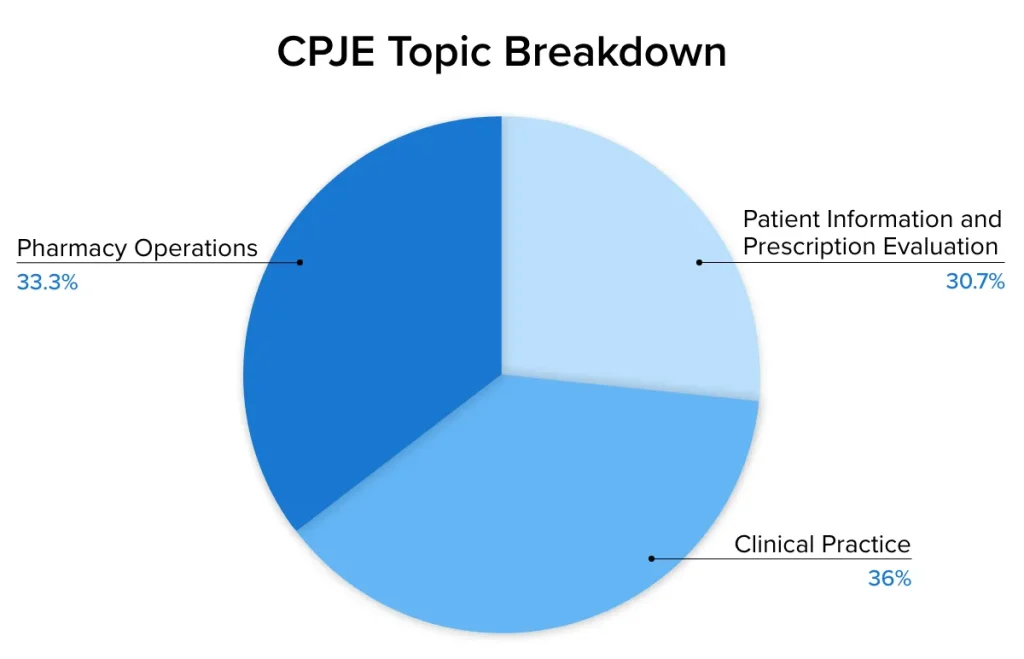
The CPJE requires candidates to show that they possess the minimum knowledge necessary to safely and effectively perform the practice of pharmacy while adhering to California jurisprudence. The CPJE content outline (i.e., blueprint) details the number of questions on the exam assigned to each main content area. The chart below outlines the question breakdown by content area:
How is the CPJE different from the MPJE?
The CPJE contains questions on clinical practice in addition to laws and regulations specific to California, whereas the MPJE only focuses on the application of the laws that govern pharmacy practice in a given jurisdiction. The clinical practice portion includes, but is not limited to, knowledge of brand and generic medications, developing therapeutic drug plans, recognizing adverse reactions, monitoring pharmacotherapy, and performing patient counseling. The CPJE content outline below provides the detailed statements of what is expected from candidates as it relates to clinical practice. Given the heavy focus on clinical content, it is prudent to use review materials that include a detailed review of clinical topics (e.g. the UWorld RxPrep NAPLEX Online Course) in addition to law materials.
CPJE Content outline
Below is the California State Board of Pharmacy content outline.
- PATIENT INFORMATION AND PRESCRIPTION EVALUATION: PRACTICE STANDARDS AND JURISPRUDENCE
- Obtain information from the patient/patient’s representative for patient profile (e.g., diagnosis or desired therapeutic outcome, allergies, adverse drug reactions, medical & medication history)
- Obtain information from prescriber and/or health care professionals for patient profile (e.g., diagnosis or desired therapeutic outcome, allergies, adverse drug reactions, medical & medication history)
- Obtain information from the patient’s medical record for patient profile (e.g., diagnosis or desired therapeutic outcome, allergies, adverse drug reactions, medical & medication history)
- Assess prescription/medication order for completeness, correctness, authenticity, and legality
- Assess prescription/medication order for appropriateness (e.g., drug selection, dosage, drug interactions, dosage form, delivery system)
- Evaluate the medical record/patient profile for any or all of the following: disease states, clinical condition, medication use, allergies, adverse drug reactions, disabilities, medical/surgical therapies, laboratory findings, physical assessments and/or diagnostic tests
- Perform physical assessment (e.g., vital signs/blood pressure measurement, observations of signs/symptoms)
- Perform health screening (e.g., blood glucose checks, diagnostic tests)
- Evaluate the pharmaceutical information needs of the patient/patient’s representative
- Determine the appropriate product(s) to be dispensed for a prescription/medication order
- Document preparation of medication in various dosage forms (e.g., compounding, repackaging)
- Meet the regulatory requirements associated with preparation and dispensing of controlled substances
- Verify label(s) for prescription containers
- Perform the final check for medications, products, preparations, or devices prior to dispensing
- Use automated drug delivery systems
- Administer immunizations
- Administer medications and biologicals as ordered by a prescriber, per protocol, or scope of practice
- Participate in compounding (sterile and non-sterile)
- CLINICAL PRACTICE: PRACTICE STANDARDS AND JURISPRUDENCE Total: 27
- Develop a therapeutic regimen for prescription medications (e.g., recommend alteration of prescribed drug regimen, select drug if necessary, perform medication therapy management)
- Determine appropriateness of non-prescription medications as part of a treatment plan (e.g., product selection, duration of therapy)
- Determine appropriateness of non-drug therapy as part of a treatment plan (e.g., selfcare, lifestyle modification)
- Collaborate with health care team and patient to determine goals of therapy and course of action
- Assess changes in health status (e.g., onset of new disease states, changes in clinical condition)
- Perform monitoring and therapeutic management activities
- Manage drug therapy according to protocols or scope of practice
- Use a formulary system (e.g., therapeutic conversions and interchanges, advising patients and prescribers, tier/formulary restrictions, insurance formularies, evaluation of products, guidelines)
- Resolve problems that arise with patient’s therapy (e.g., adverse drug reactions, drug interactions, non-adherence)
- Make evidence-based pharmacotherapy decisions
- Assess patient for immunization needs
- Resolve problems with medication affordability (e.g., insurance coverage, patient assistance programs, manufacturer cost reduction program)
- Perform medication reconciliation (including high-risk hospitalized patients)
- Recommend/order necessary monitoring procedures(e.g.,renal/hepatic function, glucose levels, EKG, drug levels)
- Initiate pharmacist-provider therapies (e.g., immunization, hormonal contraceptives, smoking cessation, travel-related medications)
- Assess the patient’s/patient representative’s understanding of health information (e.g., disease state, prevention, treatment plan)
- Counsel patient/patient representative regarding prescription medication therapy and devices
- Counsel patient/patient representative regarding nonprescription medication (OTC)
- Counsel patient/patient representative regarding herbal/complementary/alternative therapies
- Counsel patient/patient representative regarding non-drug therapy (e.g., self-care, lifestyle modification)
- Counsel patient/patient representative regarding self-monitoring of therapy (e.g., devices, symptoms)
- Verify the patient’s/patient representative’s understanding of the information presented
- Educate health care professionals (e.g., precepting intern pharmacists and answer drug information questions from healthcare providers/students)
- Communicate results of monitoring to patient/patient’s representative, prescriber, and/or other health care professionals
- Provide supplemental information as indicated (e.g., medication guides, CA pharmacist provided therapy)
- PHARMACY OPERATIONS: PRACTICE STANDARDS AND JURISPRUDENCE Total: 25
- Ensure quality specifications for pharmaceuticals, durable medical equipment, devices, and supplies (e.g., sourcing, track and trace, storage)
- Place orders for devices, supplies, and pharmaceuticals (including controlled substances)
- Maintain a record-keeping system of items purchased/received/returned in compliance with legal requirements(e.g., dangerous drugs, devices, supplies)
- Maintain a record of controlled substances ordered, received, stored, and removed from inventory
- Dispose of expired, returned, or recalled pharmaceuticals, durable medical equipment devices, supplies, and document actions taken
- Respond to changes in product availability (e.g., drug shortages, recalls)
- Prepare for and respond to emergency situations
- Design and implement policies to prevent theft and/or drug diversion
- Assess competence of pharmacy personnel (e.g., pharmacists, technicians, interns, clerks)
- Ensure the accuracy of medication administration (e.g., infusion pump reports, barcode medication administration, device re-education)
- Participate in a system to monitor quality measures and improve patient outcomes (e.g., medication use evaluations, medication error reduction program)
- Participate in a system to monitor/improve medication use including quality assurance programs (e.g., opioid stewardship, standard ordersets, peer review, self-evaluation)
- Participate in a system for medication error prevention, assessment, and reporting (e.g., root cause analysis, National Patient Safety Goals, medication error reduction program)
- Participate in a system by which adverse drug reactions and interactions are prevented, documented, evaluated, and reported
- Monitor the practice site and/or service area for compliance with federal, state, and local laws, regulations, and professional standards/guidelines
- Supervise the work of pharmacy personnel (on-site and remote)
- Ensure the availability, control, and confidentiality of patient and prescription information (e.g., patient profiles, medication administration records)
- Participate in the development of pharmacy policies and procedures, protocols, order sets, and/or therapeutic guidelines
- Participate in the use of pharmacy information systems and technology (e.g., electronic health record, e-prescribing, automated drug dispensing systems, CURES)
*Source: pharmacy.ca.gov
CPJE Review Tips
Candidates should review the CPJE PSI candidate information bulletin, which contains examples of previous exam questions.
The UWorld RxPrep CPJE Online Course is the number one resource for CPJE exam review. Materials include:
- An up-to-date printed California Law Summary for CPJE (i.e., CPJE Course Manual) featuring illustrations, law study tips, and comprehensive explanations.
- Online CPJE question bank with over 350 CPJE practice questions that mimic the most frequently tested topics, including a timed 75-item practice exam to mimic the real CPJE
- Visual aids (charts, diagrams, tables, etc.) to simplify complex concepts.
- Ability to track your performance and identity strengths and weaknesses.
- Video lectures and additional tools to complement the course manual chapters.
Since the CPJE tests heavily on clinical content in addition to specifics on jurisprudence, a comprehensive review of clinical knowledge is recommended. We recommend using the UWorld RxPrep NAPLEX course for a review of clinical content, as the CPJE Online Course focuses on the law portion of the exam.
How long should you study?
It is recommended to schedule taking the CPJE shortly after taking the NAPLEX (e.g., 2–4 weeks). Since the CPJE tests clinical knowledge, timing the CPJE in this manner will help you keep this information at the forefront of your memory. Candidates who are licensed pharmacists outside of California may need a longer time to review both clinical and law content. The total duration for studying clinical and law content may range from 3 to 6 months, depending on the candidate. Our CPJE study guide will help you study efficiently and ace your exam.
Frequently Asked Questions (FAQs)
What should I take to the CPJE exam center?
Candidates should bring their identification. All candidates will be thumb printed, and you must present two forms of identification or you will not be permitted entry to the testing center. Examples of acceptable identification are provided in the CPJE PSI candidate information bulletin.
Food and beverages are not permitted in the testing center.
How many questions do you need to get correct to pass the CPJE?
Performance on the CPJE is measured as a scaled score ranging from 0 to 99. Candidates must achieve a scaled score of 75. Note that this does not mean that students must answer 75% of the questions correctly.
What happens if you fail the CPJE?
If you fail the CPJE, the board will give you instructions for retaking the exam, which includes submitting a retake application. You must wait 45 days before you can retake the CPJE.
How many times can you take the CPJE?
Candidates may attempt the CPJE a total of four times. If unsuccessful, the candidate is required to complete 16 semester units of education in pharmacy as approved by the board, before they are permitted to sit for the CPJE again.
Read More About the CPJE Exam
Wondering if you are eligible for the CPJE? Not sure how to register? Get all the information you need about registration costs and eligibility requirements in this article.
CPJE Study GuideOptimize your study time with our expert-curated CPJE study guide. You’ll find an in-depth CPJE blueprint that will help you ace the exam.