The following blueprint outlines the key knowledge areas and skills required for pharmacy licensure exams in individual states. The statements below are organized into 4 core areas that cover the legal and practical aspects of pharmacy practice, including licensure, personnel regulations, medication dispensing, and daily operations. Use this guide to identify where to focus your MPJE Exam preparation.
MPJE Competency Statements
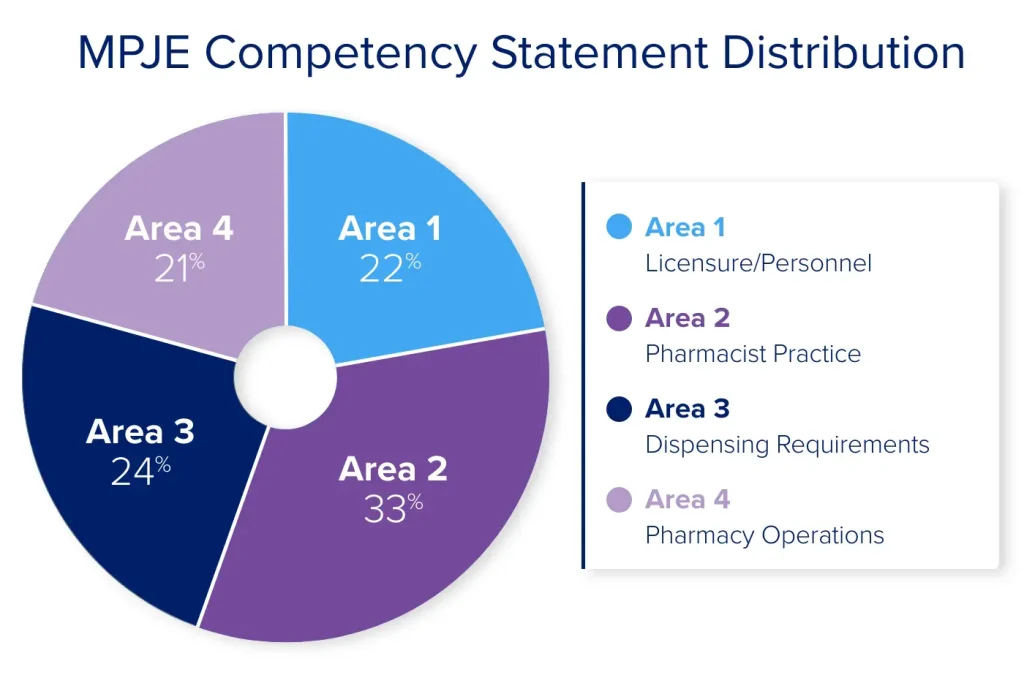
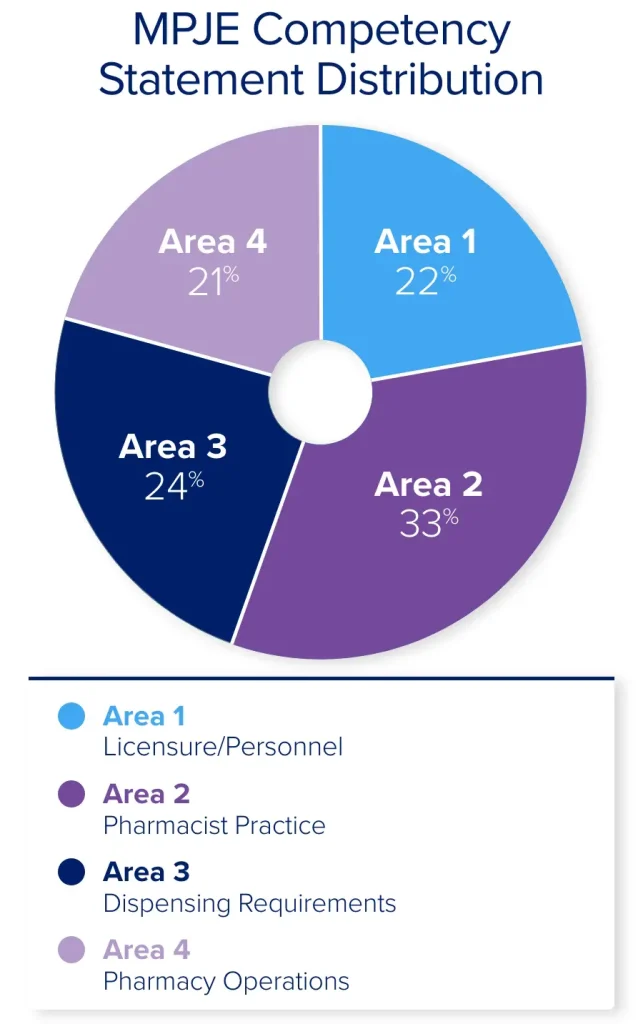
The MPJE Competency Statements describe the knowledge and skills you will need to apply to questions on your exam. This blueprint is broken down into 4 content areas: Licensure/Personnel (22%), Pharmacist Practice (33%), Dispensing Requirements (24%), and Pharmacy Operations (21%).
Area 1: Licensure/Personnel
Area 1 of the MPJE Competency Statements represents 22% of the exam content and covers topics that regulate the duties of pharmacists and non-pharmacist personnel, including licensure requirements. Specifically, you will need to be familiar with federal and state laws that outline the responsibilities of pharmacy personnel (e.g., pharmacists, interns, technicians) and define their scope of practice.
This area is further broken down into the following items:
1.1: Responsibilities of the pharmacist and non-pharmacist personnel
- 1.1.1: Qualifications, scope of duties, limitations and restrictions of duties, or conditions to practice for pharmacists-in-charge (or equivalent) and pharmacists
- 1.1.2: Qualifications, scope of duties, limitations, and restrictions of duties, or conditions to practice for non-pharmacist personnel
1.2: Licensures, registrations, and certifications for pharmacists or non-pharmacist personnel
- 1.2.1: Qualifications, examinations, internships, maintaining pharmacist competency, and renewals of licensures, registrations, or certifications
- 1.2.2: Classifications and processes of disciplinary actions
- 1.2.3: Reporting to and participating in programs addressing the inability to practice with reasonable skill and safety
Area 2: Pharmacist Practice
Area 2 of the MPJE Competency Statements represents 33% of the exam and covers dispensing requirements for controlled and non-controlled medications, including limitations on the practitioner’s authority to prescribe. This area also covers laws regarding patient counseling of medications and protecting patient confidentiality.
This area is further broken down into the following items:
2.1: Requirements for issuing prescriptions/drug orders
- 2.1.1 Requirements for drug uses, limitations, or restrictions
- 2.1.2: Scope of authority, scope of practice, limitations or restrictions of practice, and valid registration of practitioners who are authorized to prescribe, dispense, or administer drugs
- 2.1.3: Requirements for issuing non-controlled prescriptions/drug orders
- 2.1.4: Requirements for issuing controlled prescriptions/drug orders
- 2.1.5: Authority limitations of practitioners’ ability to authorize refills
2.3: Requirements regarding counseling
- 2.3.1: Counseling or offering to counsel
- 2.3.2: Documenting counseling or documenting offering to counsel
2.5: Regulations and agencies regarding pharmacy practice
- 2.5.1: Requirements for promoting quality and safety of public health
- 2.5.2: Protecting patient and health record confidentiality
Area 3: Dispensing Requirements
Area 3 of the MPJE Competency Statements represents 24% of the exam and tests on requirements for labeling and packaging of dispensed medications, transferring prescriptions, and conditions where dispensing a drug is prohibited. It also covers requirements for distributing and dispensing hazardous drugs, over-the-counter (OTC) medications, and restricted non-prescription (e.g., behind-the-counter) products.
3.3: Prospective drug utilization reviews
- 3.3.1: Requirements for reporting to PMP and accessing PMP data
3.8: Requirements for the distribution and/or dispensing of non-prescription pharmaceutical products, including controlled substances and hazardous drugs
- 3.8.1: Dispensing or administration
- 3.8.2: Labeling of non-prescription drugs and devices
- 3.8.3: Packaging and repackaging of non-prescription drugs and behind-the-counter products
- 3.8.4: Dispensing restricted, non-prescription drugs
Area 4: Pharmacy Operations
Area 4 of the MPJE Competency Statements represents 21% of the exam and tests on topics related to the day-to-day operation of a pharmacy, including ordering and acquiring drugs, recordkeeping, and compounding (e.g., hazardous and non-hazardous preparations). It also covers licensure requirements for specific pharmacy settings or businesses.
4.1: Ordering, acquisition, and distribution of drugs, including maintenance and content of such records
- 4.1.1: Ordering and acquisition, including the maintenance and content of such records
- 4.1.2: Distribution, including the maintenance and content of such records
4.2: Recordkeeping in compliance with legal requirements, including content, inventory, maintenance, storage, handling, and reporting
- 4.2.1: Non-dispensing requirements for operations of pharmacies or practice settings
- 4.2.2: Possession, storage, and handling of non-hazardous drugs
- 4.2.3: Training, possession, handling, storage, and disposal of hazardous drugs
- 4.2.4: Allowing non-pharmacist personnel access to drugs
- 4.2.5: Requirements for conducting controlled substance inventories
4.7: Requirements for the registration, licensure, certification, or permitting of a practice setting or business entity
- 4.7.1: Requirements for registration, license, certification, or permitting of a practice setting
- 4.7.2: Requirements for the renewal or reinstatement of a license, registration, certificate, or permit of a practice setting
- 4.7.3: Requirements for an inspection of a licensed, registered, certified, or permitted practice setting
- 4.7.4: Classifications and processes of disciplinary actions that may be taken against a registered, licensed, certified, or permitted practice setting
How to Pass the MPJE Exam Using the Blueprint and UWorld RxPrep
Use this MPJE blueprint to help you plan your exam preparation, make your own study schedule, and prepare yourself so that there are no surprises on exam day. Plan one section at a time, as opposed to all four content areas at once. This will help you concentrate your study efforts.
Our recommendation would be to prepare for difficult topics first, as it is a time-intensive process. You can save time by studying the MPJE Competency Statements according to their weight on the exam. This will enable you to dedicate an appropriate amount of time to each topic.
It’s important to note that you cannot solely rely on the MPJE competency statements. Pairing the MPJE Competency Statements with a strong, comprehensive review course is key to passing the MPJE on your first try. The UWorld RxPrep MPJE Online Course covers each topic on the blueprint and emphasizes areas where state laws are stricter than federal law. The course includes a course manual with a 9-page fill-in-the-blank study guide, a QBank with 225+ questions, targeted video lectures, time-saving study tools and 110+ state-specific online flashcards.
Read More About the MPJE
Learn everything you need to know about the MPJE, including the registration process and exam format.
MPJE Study GuideRead our expert study guide for MPJE resources and study tips that increase your chances of passing the exam.
MPJE Scoring GuideGet details about the MPJE's passing criteria, as well as score transfers, rescore procedures, and exam retakes.
MPJE Registration, Cost, and EligibilityLearn how to register, eligibility requirements, and the costs to apply for the MPJE





