Free Premium MPJE® Practice Questions
Practice with questions written by pharmacists that mirror exam-level difficulty. If practice feels like the exam, the real thing will feel like practice.
MPJE Sample Practice Questions
The pharmacist receives a new prescription for Ritalin and notices that the prescriber’s signature is missing. What should the pharmacist do?
| A. | Fix the error without consulting with the prescriber. | ||
| B. | Fix the error after consulting with the prescriber. | ||
| C. | Fix the error after consulting with an agent of the prescriber. | ||
| D. | Return the prescription to the patient to have the prescription amended by the prescriber. | ||
| E. | Call the prescriber for a new, oral prescription. |
Explanation:
Without the prescriber’s signature, the prescription is invalid.
Textbook References :
- Refer to the latest version of the RxPrep Course Book – Controlled Substances Part 3 chapter, “Errors or Omissions”, beginning on page 141.
Lecture References :
- Errors or Omissions
Which of the following providers do not need to register with the DEA to prescribe controlled substances? (Select ALL that apply.)
| A. | Medical residents at a hospital registered with the DEA | ||
| B. | Physicians at a Navy hospital | ||
| C. | All providers must register with the DEA to prescribe controlled substances | ||
| D. | Physicians at a methadone clinic | ||
| E. | Nurse practitioners at the Federal Bureau of Prisons |
Explanation:
A controlled drug may be prescribed by employees (e.g., medical residents) working under the DEA registration of a healthcare facility. Individuals that are exempt from registering for a personal DEA number include healthcare workers in the military, Public Health Service or Federal Bureau of Prisons.
Textbook References :
- Refer to the latest version of the RxPrep Course Book – Controlled Substances Part 2 chapter, “Controlled Substance Registrants”, beginning on page 118.
Lecture References :
- Controlled Substance Registrants
Which of the following can be inventoried with an estimated count?
| A. | Opened bottles of Concerta, < 100 tablets/bottle | ||
| B. | Unopened bottles of Marinol, 60 capsules/bottle | ||
| C. | Opened bottle of Provigil, < 30 tablets/bottle | ||
| D. | Unopened bottle of Tylenol with Codeine #4, 500 tablets/bottle | ||
| E. | Opened bottles of meperidine, < 500 tablets/bottle |
Explanation:
For sealed, unopened containers of all controlled substances, an exact count is needed. For opened containers of schedule I and II, an exact count is required. For opened containers of schedule III-V, an exact count is required only if the container holds > 1,000 dosage units.
Textbook References :
- Refer to the latest version of the RxPrep Course Book – Controlled Substances Part 2 chapter, “DEA Controlled Substances Inventory Requirements”, beginning on page 129.
Lecture References :
- DEA Controlled Substances Inventory Requirements
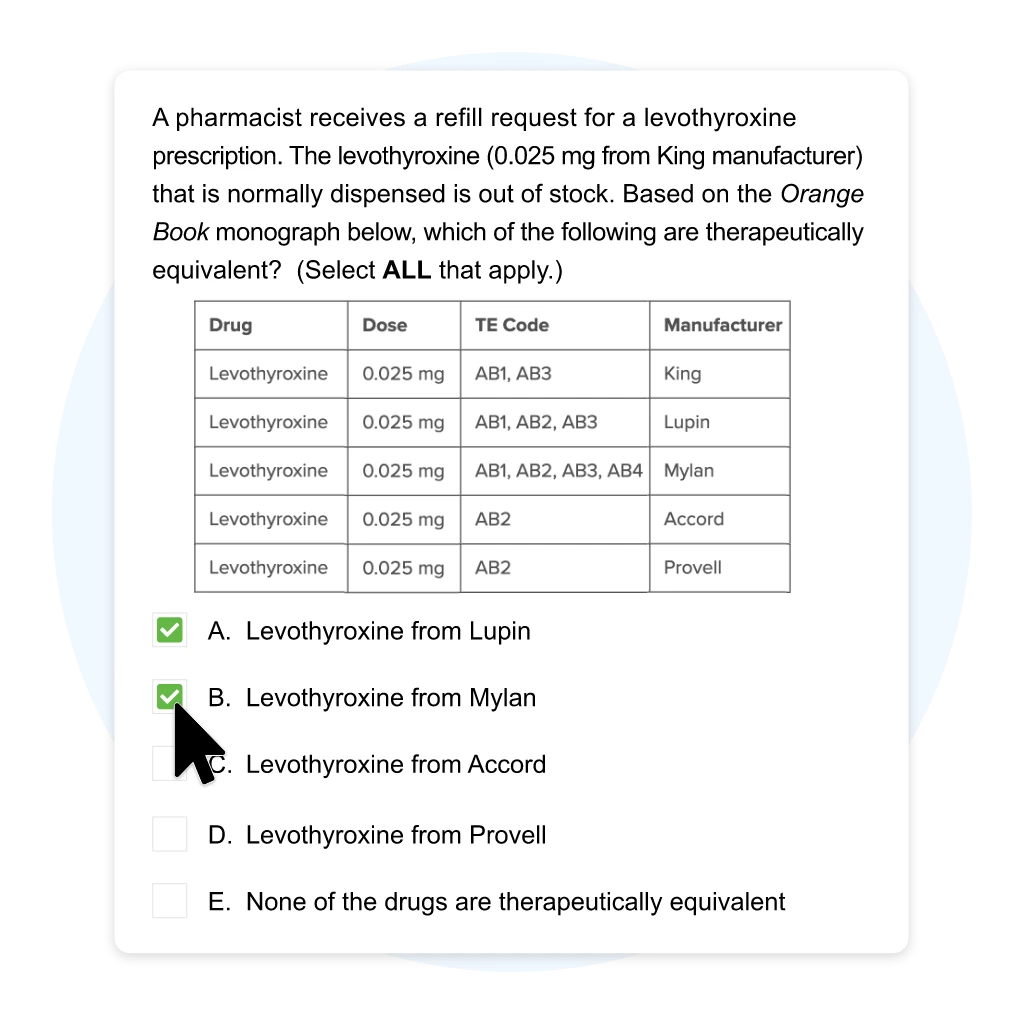
A pharmacist receives a refill request for a levothyroxine prescription. The levothyroxine (0.025 mg from King manufacturer) that is normally dispensed is out of stock. Based on the Orange Book monograph below, which of the following are therapeutically equivalent? (Select ALL that apply.)
| Drug | Dose | TE Code | Manufacturer |
|---|---|---|---|
| Levothyroxine | 0.025 mg | AB1, AB3 | King |
| Levothyroxine | 0.025 mg | AB1, AB2, AB3 | Lupin |
| Levothyroxine | 0.025 mg | AB1, AB2, AB3, AB4 | Mylan |
| Levothyroxine | 0.025 mg | AB2 | Accord |
| Levothyroxine | 0.025 mg | AB2 | Provell |
| A. | Levothyroxine from Lupin | ||
| B. | Levothyroxine from Mylan | ||
| C. | Levothyroxine from Accord | ||
| D. | Levothyroxine from Provell | ||
| E. | None of the drugs are therapeutically equivalent |
Explanation:
Levothyroxine has many reference listed drugs (RLD) and a three-character code is used in the Orange Book. The levothyroxine that the pharmacy normally dispenses has a therapeutic equivalence (TE) code of AB1 and AB3. It is therapeutically equivalent to other levothyroxine with the same codes (i.e., levothyroxine from Lupin and Mylan).
Textbook References :
- Refer to the latest version of the RxPrep Course Book – Pharmacy Practice chapter, “The Orange Book”, beginning on page 76.
Lecture References :
- The Orange Book
Which of the following medications is misbranded?
| A. | A unit dose label for Invokana does not have a beyond-use date on it. | ||
| B. | A Pepcid tablet containing glass shards in the tablet. | ||
| C. | An elixir manufactured with a toxic, unapproved ingredient. | ||
| D. | A methylprednisolone injection found to be growing mold. | ||
| E. | None of the answers are correct. |
Explanation:
Misbranding (labeling) is the lack of required information on the package and in the labeling or the information is illegible (cannot be read), false or misleading information, imitating another drug or promising false cures, missing special precautions needed to prevent decomposition (e.g., protect from light), improper packaging (i.e., lack of Poison Prevention Packaging) or incomplete labeling of additives. Additionally, if the ingredients differ from the standard of strength, quality, or purity (as determined by USP) the product is considered misbranded.
Textbook References :
- Refer to the latest version of the RxPrep Course Book – Pharmacy Laws and Regulations chapter, “Food, Drug and Cosmetic Act of 1938”, beginning on page 14.
Lecture References :
- Food, Drug and Cosmetic Act of 1938
The new pharmacist-in-charge at a community pharmacy would like to reorganize the drug stock. Which of the following are acceptable ways to store controlled substances? (Select ALL that apply.)
| A. | Schedule II controlled substances stored in a securely locked cabinet | ||
| B. | All controlled substances stored in a securely locked vault | ||
| C. | All controlled substances in an unlocked storage box, well-hidden from view | ||
| D. | All controlled substances together on a designated shelf that is not facing the customer service area | ||
| E. | Schedule III-V controlled substances dispersed throughout the drug stock with non-controlled drugs on the shelves |
Explanation:
Under federal law, schedule II-V drugs can be concealed by dispersing them throughout the non-controlled substances on the shelves. Many states only allow schedule III-V drugs to be dispersed among non-controlled stock. Otherwise, controlled substances must be stored in a securely locked cabinet of “substantial construction.”
Textbook References :
- Refer to the latest version of the RxPrep Course Book – Pharmacy Operations chapter, “Controlled Substance Storage”, beginning on page 44.
Lecture References :
- Controlled Substance Storage
Want to Keep Practicing?
Explore MPJE Question Format
Why More Students Choose UWorld RxPrep
Exam-Like Practice
Exam-Like
Practice
State-specific Content
Customizable Practice Tests
Join the Thousands Who Will Pass with UWorld RxPrep
Learn More about the MPJE

MPJE Blueprint
A blueprint of the NABP’s MPJE competency statement content and distribution.

MPJE Information
An in-depth overview of MPJE eligibility, structure, content areas, and prep tips.

MPJE Study Guide
A comprehensive guide detailing MPJE study materials, techniques, and schedules.